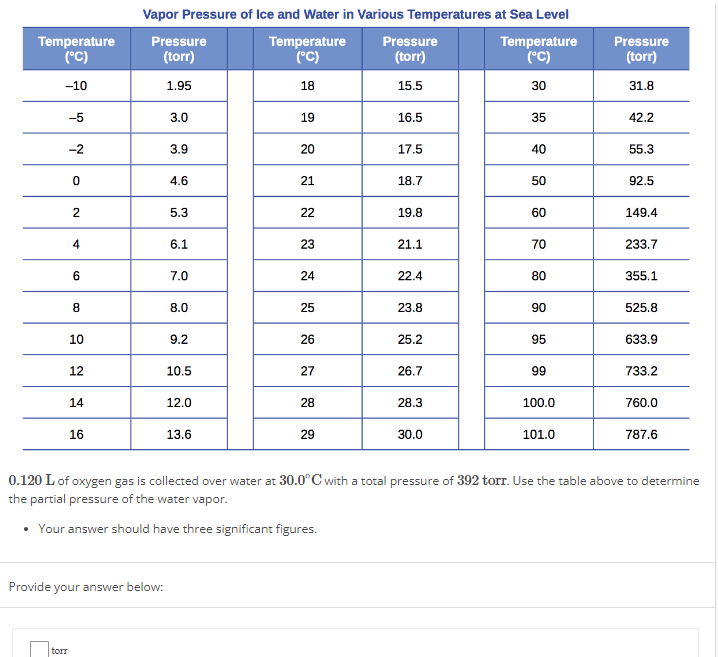
0.120 L of oxygen gas is collected over water at 30.0∘C with a total pressure of 392 torr. Use the table above to determine the partial pressure of the water vapor. Your answer should have three significant figures. Provide your answer below: Vapor Pressure of Ice and Water in Various Temperatures at Sea Level