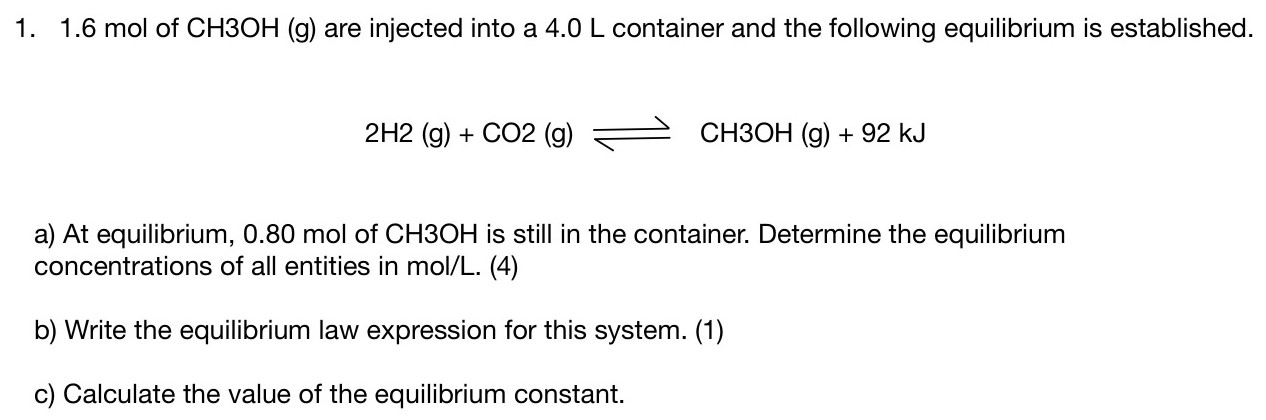
1.6 mol of CH3OH (g) are injected into a 4.0 L container and the following equilibrium is established. 2H2 (g) + CO2 (g) ⇌ CH3OH (g) + 92 kJ a) At equilibrium, 0.80 mol of CH3OH is still in the container. Determine the equilibrium concentrations of all entities in mol/L. (4) b) Write the equilibrium law expression for this system. (1) c) Calculate the value of the equilibrium constant.