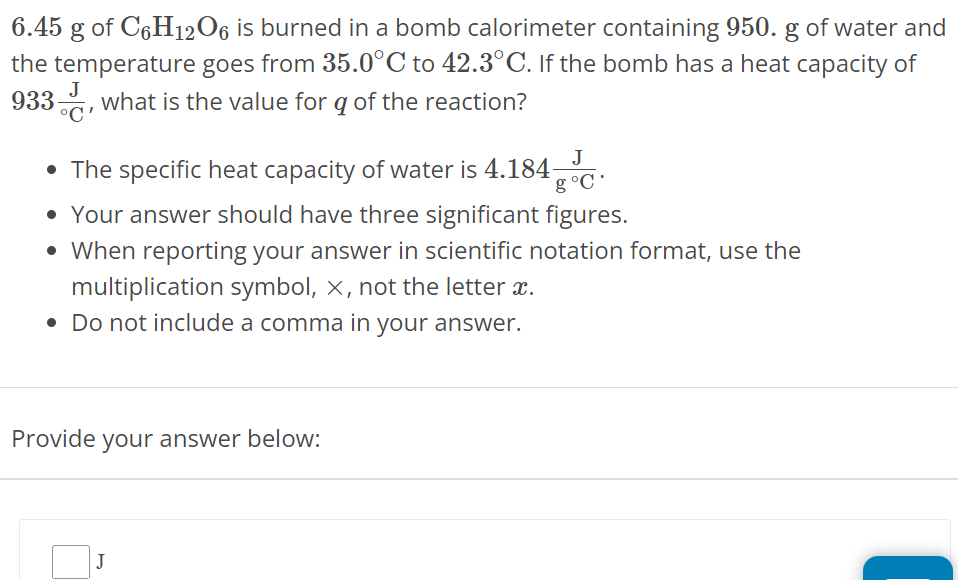
6.45 g of C6H12O6 is burned in a bomb calorimeter containing 950. g of water and the temperature goes from 35.0∘C to 42.3∘C. If the bomb has a heat capacity of 933 J∘C, what is the value for q of the reaction? The specific heat capacity of water is 4.184 J g∘C. Your answer should have three significant figures. When reporting your answer in scientific notation format, use the multiplication symbol, ×, not the letter x. Do not include a comma in your answer. Provide your answer below: