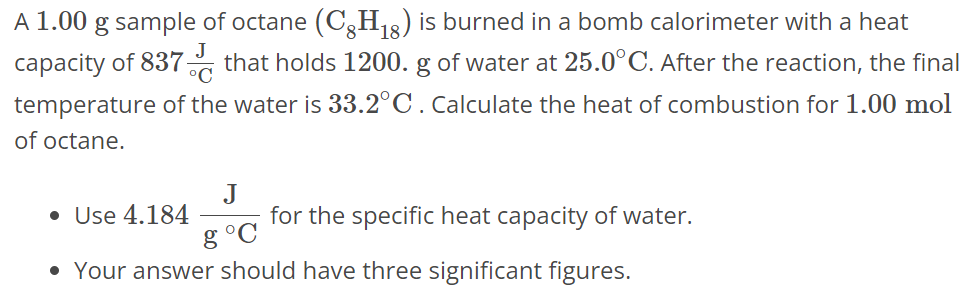
A 1.00 g sample of octane (C8H18) is burned in a bomb calorimeter with a heat capacity of 837 J∘C that holds 1200. g of water at 25.0∘C. After the reaction, the final temperature of the water is 33.2∘C. Calculate the heat of combustion for 1.00 mol of octane. Use 4.184 J g∘C for the specific heat capacity of water. Your answer should have three significant figures.


You'll get a detailed, step-by-step and expert verified solution.
 Work With Experts to Reach at Correct Answers
Work With Experts to Reach at Correct Answers