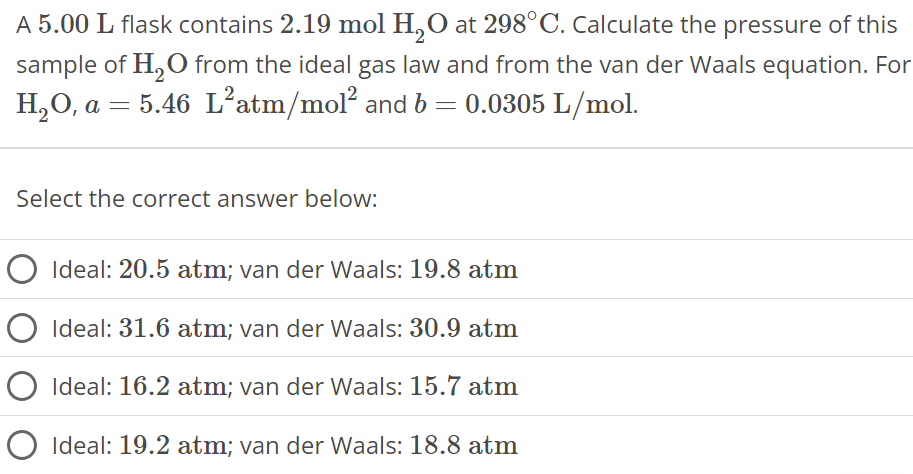
A 5.00 L flask contains 2.19 mol H2O at 298∘C. Calculate the pressure of this sample of H2O from the ideal gas law and from the van der Waals equation. For H2O, a = 5.46 L2 atm/mol2 and b = 0.0305 L/mol. Select the correct answer below: Ideal: 20.5 atm; van der Waals: 19.8 atm Ideal: 31.6 atm; van der Waals: 30.9 atm Ideal: 16.2 atm; van der Waals: 15.7 atm Ideal: 19.2 atm; van der Waals: 18.8 atm