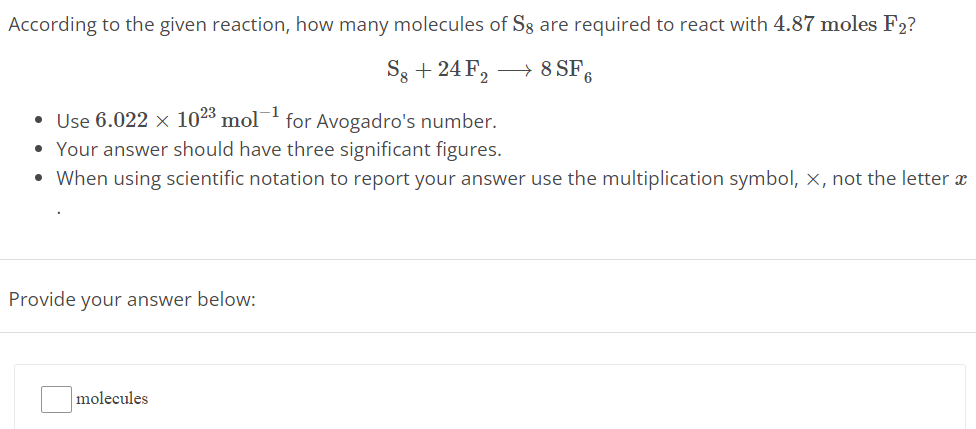
According to the given reaction, how many molecules of S8 are required to react with 4.87 moles F2 ? S8 + 24F2 ⟶ 8SF6 Use 6.022×1023 mol−1 for Avogadro's number. Your answer should have three significant figures. When using scientific notation to report your answer use the multiplication symbol, ×, not the letter x Provide your answer below: molecules