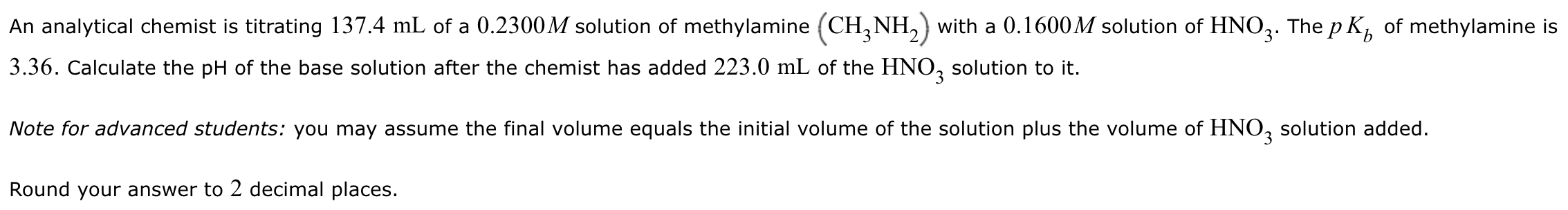
An analytical chemist is titrating 137.4 mL of a 0.2300 M solution of methylamine (CH3NH2) with a 0.1600 M solution of HNO3. The pKb of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 223.0 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places.