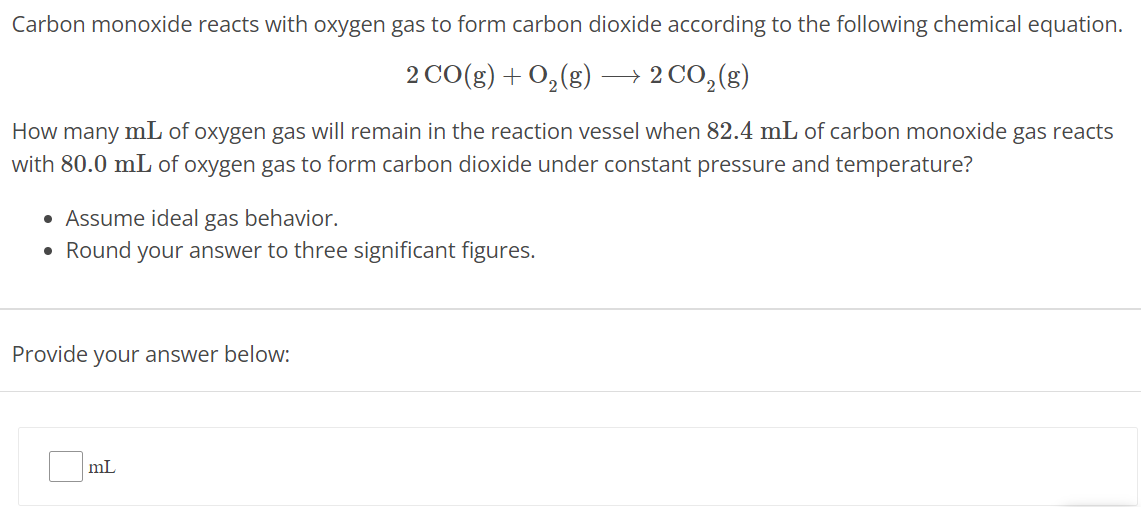Carbon monoxide reacts with oxygen gas to form carbon dioxide according to the following chemical equation. 2 CO(g) + O2(g) ⟶ 2 CO2(g) How many mL of oxygen gas will remain in the reaction vessel when 82.4 mL of carbon monoxide gas reacts with 80.0 mL of oxygen gas to form carbon dioxide under constant pressure and temperature? Assume ideal gas behavior. Round your answer to three significant figures. Provide your answer below: mL