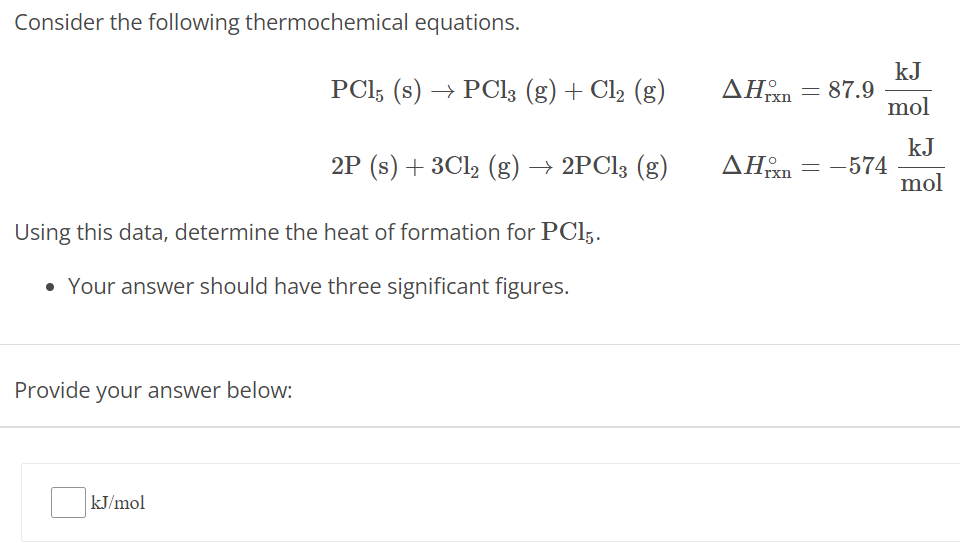
Consider the following thermochemical equations. PCl5(s) → PCl3(g) + Cl2(g) ΔHrxn∘ = 87.9 kJ mol 2P(s) + 3Cl2(g) → 2PCl3(g) ΔHrxn∘ = −574 kJ mol Using this data, determine the heat of formation for PCl5. Your answer should have three significant figures. Provide your answer below: kJ/mol