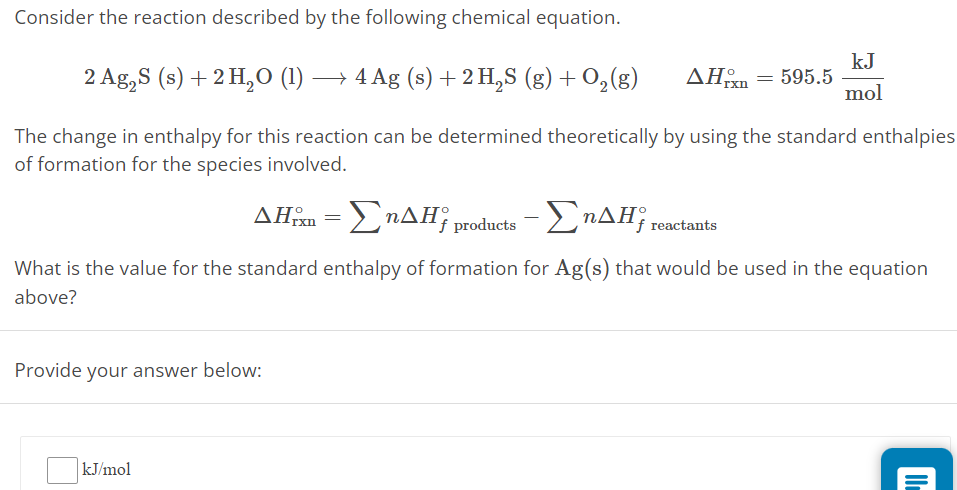
Consider the reaction described by the following chemical equation. 2 Ag2S(s) + 2 H2O(l) ⟶ 4 Ag(s) + 2 H2S(g) + O2(g) ΔHrxn∘ = 595.5 kJ mol The change in enthalpy for this reaction can be determined theoretically by using the standard enthalpies of formation for the species involved. ΔHrxn∘ = ∑nΔHf products ∘−∑nΔHf reactants ∘ What is the value for the standard enthalpy of formation for Ag(s) that would be used in the equation above? Provide your answer below: kJ/mol