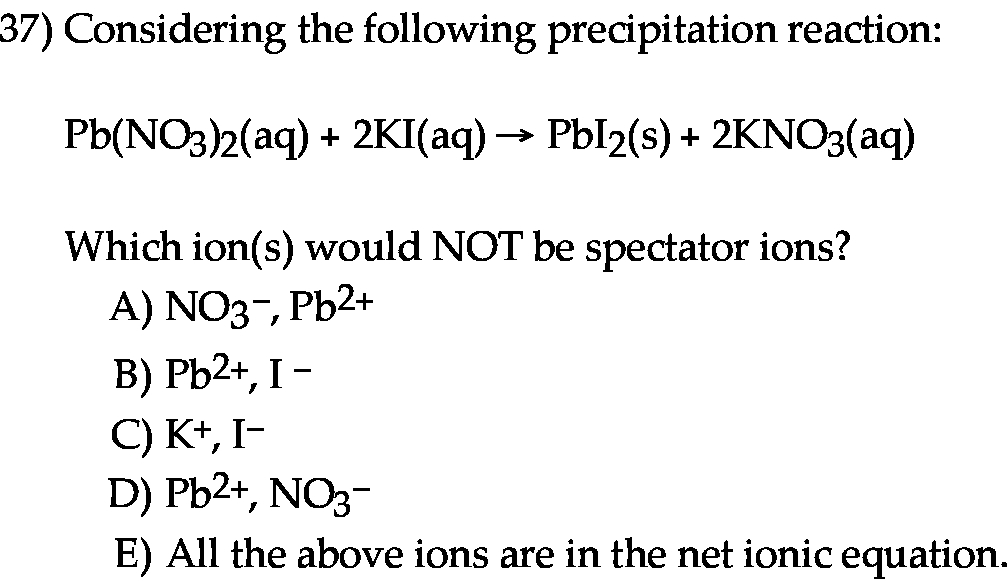
Considering the following precipitation reaction: Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq) Which ion(s) would NOT be spectator ions? A) NO3−, Pb2+ B) Pb2+, I− C) K+, I− D) Pb2+, NO3− E) All the above ions are in the net ionic equation.


You'll get a detailed, step-by-step and expert verified solution.
 Work With Experts to Reach at Correct Answers
Work With Experts to Reach at Correct Answers