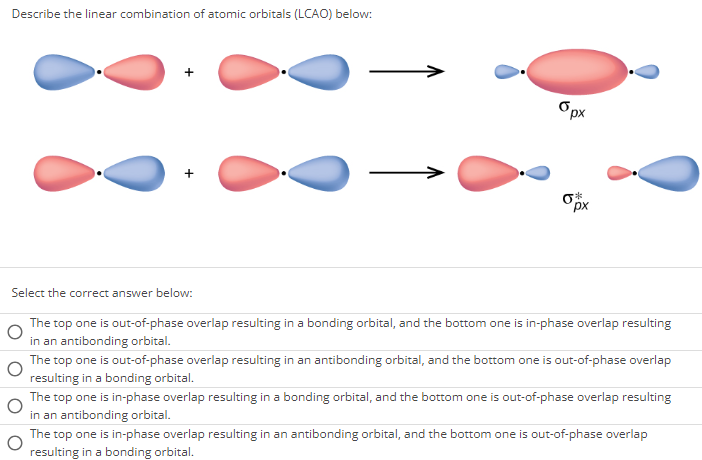
Describe the linear combination of atomic orbitals (LCAO) below: Select the correct answer below: The top one is out-of-phase overlap resulting in a bonding orbital, and the bottom one is in-phase overlap resulting in an antibonding orbital. The top one is out-of-phase overlap resulting in an antibonding orbital, and the bottom one is out-of-phase overlap resulting in a bonding orbital. The top one is in-phase overlap resulting in a bonding orbital, and the bottom one is out-of-phase overlap resulting in an antibonding orbital. The top one is in-phase overlap resulting in an antibonding orbital, and the bottom one is out-of-phase overlap resulting in a bonding orbital.