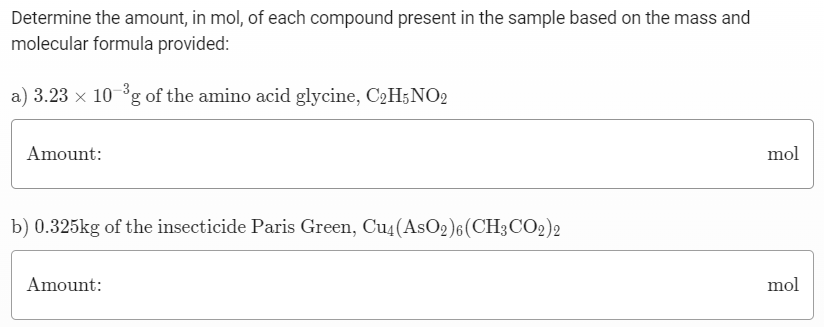
Determine the amount, in mol, of each compound present in the sample based on the mass and molecular formula provided: a) 3.23×10−3 g of the amino acid glycine, C2H5NO2 Amount: mol b) 0.325 kg of the insecticide Paris Green, Cu4(AsO2)6(CH3 CO2)2 Amount: mol