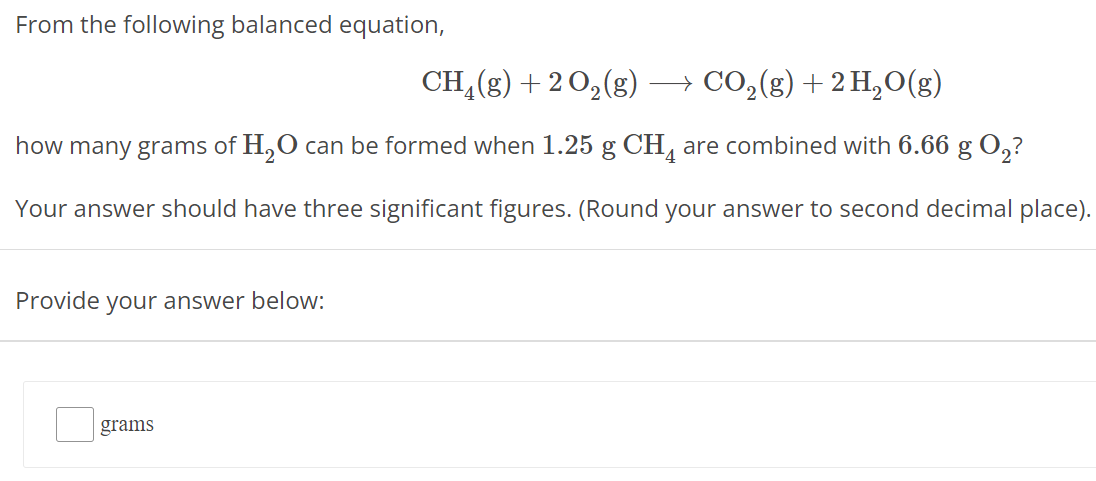
From the following balanced equation, CH4(g) + 2 O2(g) ⟶ CO2(g) + 2 H2O(g) how many grams of H2O can be formed when 1.25 g CH4 are combined with 6.66 g O2 ? Your answer should have three significant figures. (Round your answer to second decimal place). Provide your answer below: grams


You'll get a detailed, step-by-step and expert verified solution.
 Work With Experts to Reach at Correct Answers
Work With Experts to Reach at Correct Answers