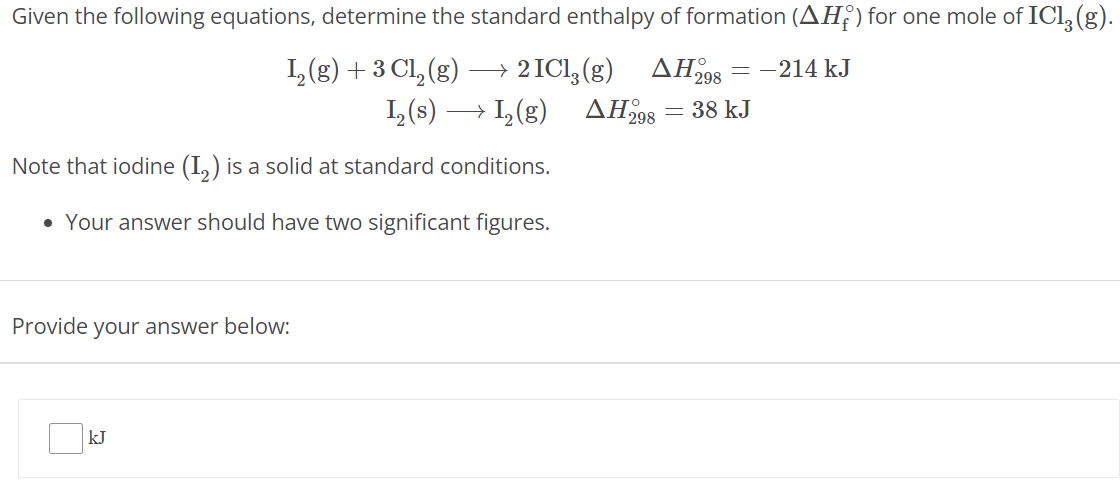
Given the following equations, determine the standard enthalpy of formation (ΔHf∘) for one mole of ICl3(g). I2(g) + 3 Cl2(g) ⟶ 2 ICl3(g) ΔH298∘ = −214 kJ I2(s) ⟶ I2(g) ΔH298∘ = 38 kJ Note that iodine (I2) is a solid at standard conditions. Your answer should have two significant figures. Provide your answer below: kJ