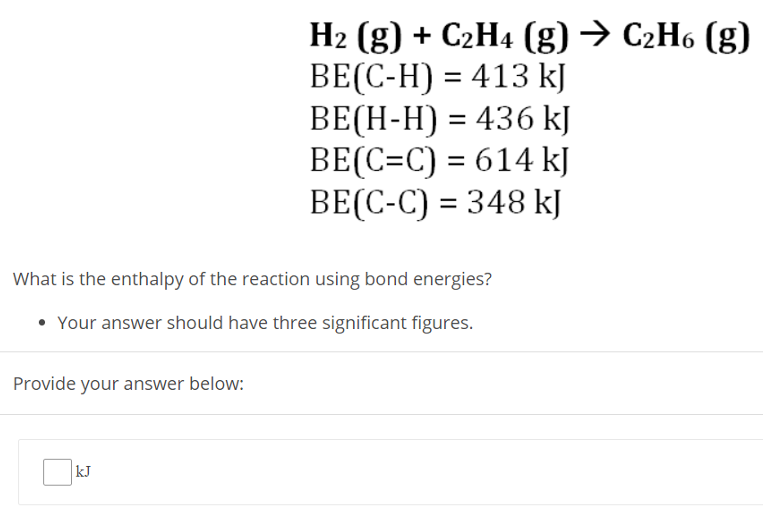
H2 (g) + C2H4 (g) → C2H6 (g) BE(C−H) = 413 kJ BE(H−H) = 436 kJ BE(C = C) = 614 kJ BE(C−C) = 348 kJ What is the enthalpy of the reaction using bond energies? Your answer should have three significant figures. Provide your answer below: kJ


You'll get a detailed, step-by-step and expert verified solution.
 Work With Experts to Reach at Correct Answers
Work With Experts to Reach at Correct Answers