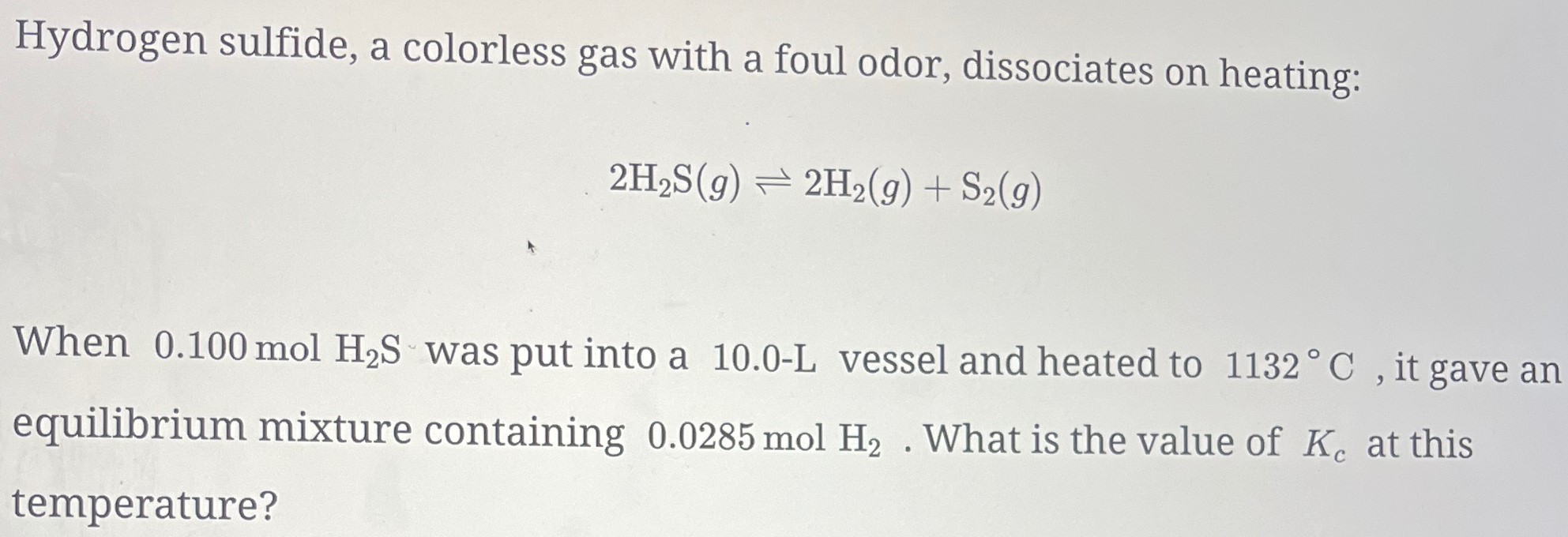Hydrogen sulfide, a colorless gas with a foul odor, dissociates on heating: 2H2S(g) ⇌ 2H2(g) + S2(g) When 0.100 mol H2S was put into a 10.0−L vessel and heated to 1132∘C, it gave an equilibrium mixture containing 0.0285 mol H2. What is the value of Kc at this temperature?