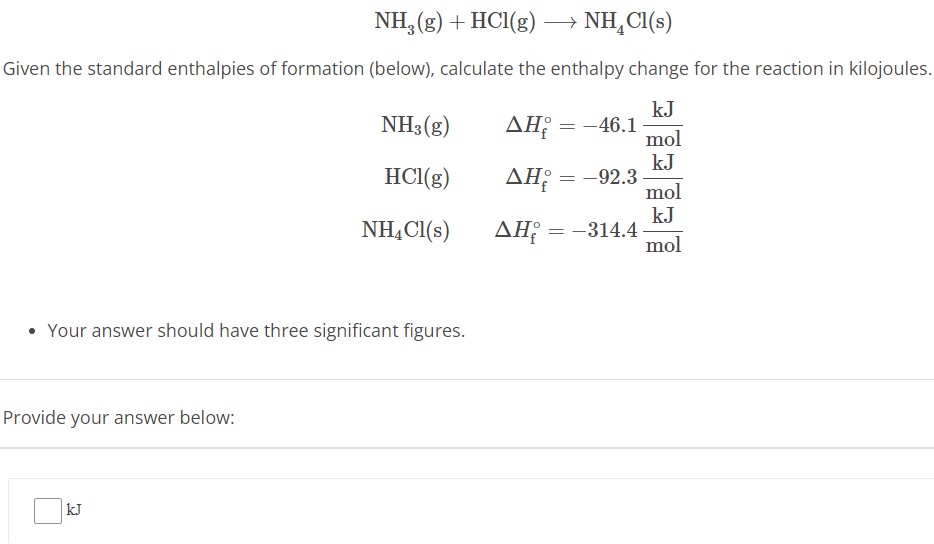
NH3(g) + HCl(g) ⟶ NH4Cl(s) Given the standard enthalpies of formation (below), calculate the enthalpy change for the reaction in kilojoules. NH3(g) ΔHf∘ = −46.1 kJ mol HCl(g) ΔHf∘ = −92.3 kJ mol NH4Cl(s) ΔHf∘ = −314.4 kJ mol Your answer should have three significant figures. Provide your answer below: kJ


You'll get a detailed, step-by-step and expert verified solution.
 Work With Experts to Reach at Correct Answers
Work With Experts to Reach at Correct Answers