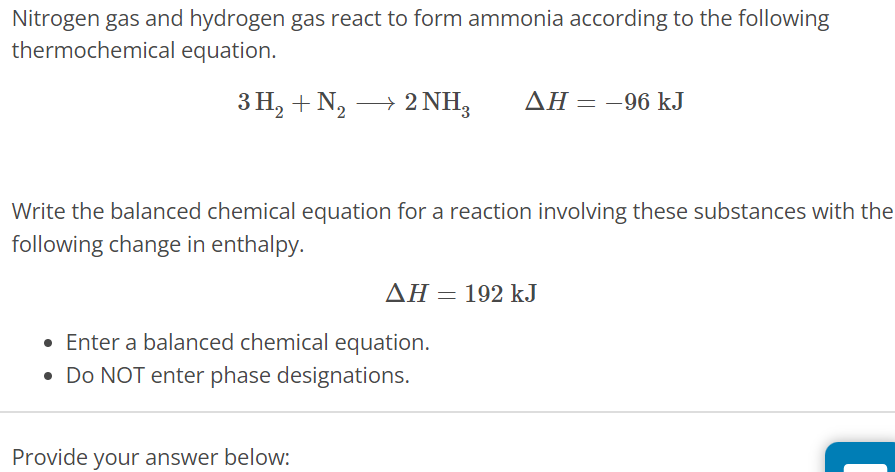
Nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. 3H2 + N2 ⟶ 2NH3 ΔH = −96 kJ Write the balanced chemical equation for a reaction involving these substances with the following change in enthalpy. ΔH = 192 kJ Enter a balanced chemical equation. Do NOT enter phase designations. Provide your answer below: