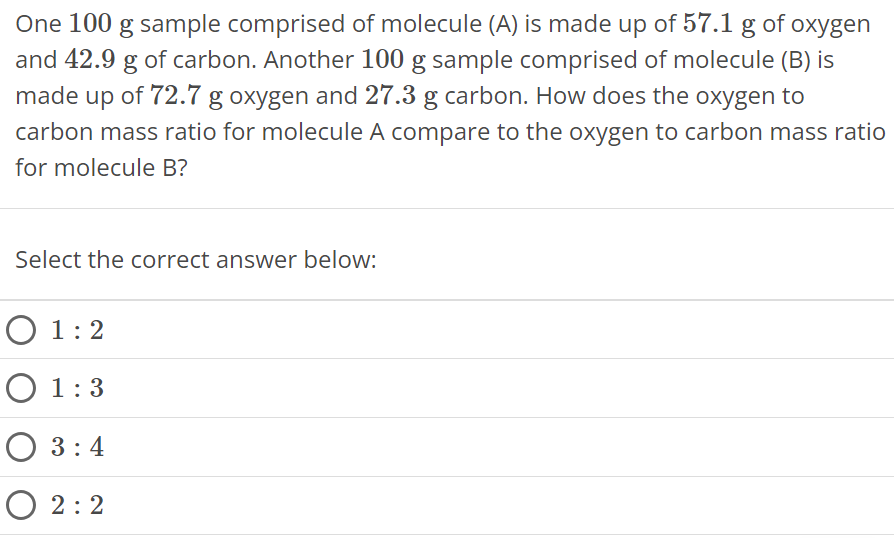One 100 g sample comprised of molecule (A) is made up of 57.1 g of oxygen and 42.9 g of carbon. Another 100 g sample comprised of molecule (B) is made up of 72.7 g oxygen and 27.3 g carbon. How does the oxygen to carbon mass ratio for molecule A compare to the oxygen to carbon mass ratio for molecule B? Select the correct answer below: 1 : 2 1 : 3 3 : 4 2 : 2