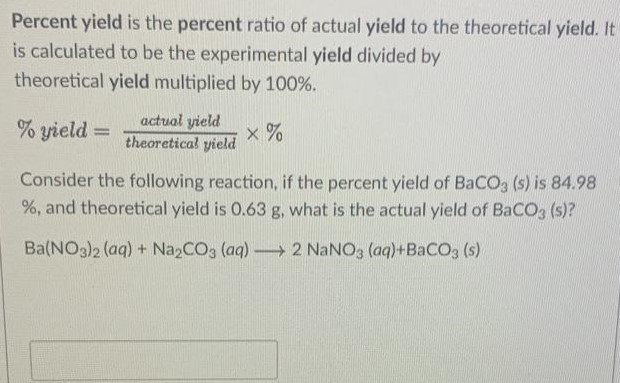Percent yield is the percent ratio of actual yield to the theoretical yield. It is calculated to be the experimental yield divided by theoretical yield multiplied by 100%. % yield = actual yield theoretical yield ×% Consider the following reaction, if the percent yield of BaCO3(s) is 84.98 %, and theoretical yield is 0.63 g, what is the actual yield of BaCO3(s) ? Ba(NO3)2(aq) + Na2CO3(aq) ⟶ 2NaNO3(aq) + BaCO3(s)