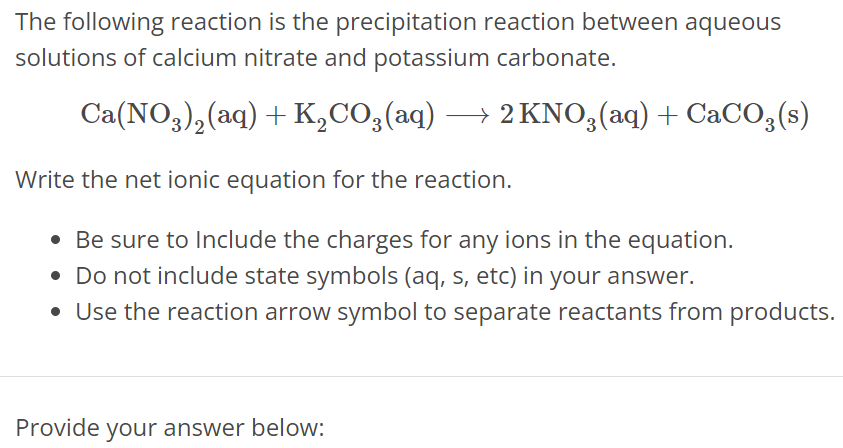
The following reaction is the precipitation reaction between aqueous solutions of calcium nitrate and potassium carbonate. Ca(NO3)2(aq) + K2CO3(aq) ⟶ 2KNO3(aq) + CaCO3(s) Write the net ionic equation for the reaction. Be sure to Include the charges for any ions in the equation. Do not include state symbols (aq, s, etc) in your answer. Use the reaction arrow symbol to separate reactants from products. Provide your answer below: