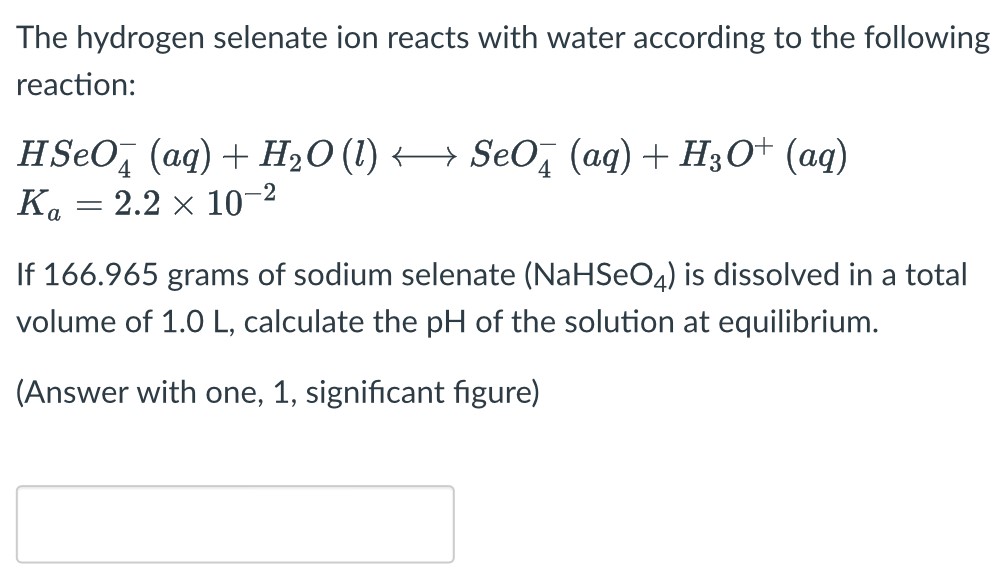
The hydrogen selenate ion reacts with water according to the following reaction: HSeO4−(aq) + H2O(l) ⟷ SeO4−(aq) + H3O+(aq) Ka = 2.2×10−2 If 166.965 grams of sodium selenate (NaHSeO4) is dissolved in a total volume of 1.0 L, calculate the pH of the solution at equilibrium. (Answer with one, 1, significant figure)