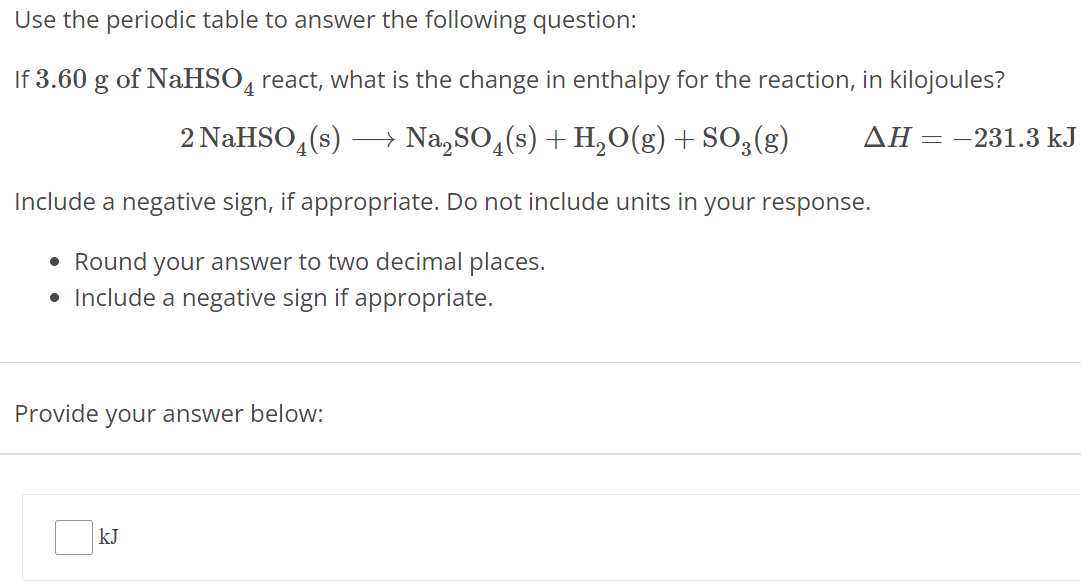
Use the periodic table to answer the following question: If 3.60 g of NaHSO4 react, what is the change in enthalpy for the reaction, in kilojoules? 2 NaHSO4(s) ⟶ Na2SO4(s) + H2O(g) + SO3(g) ΔH = −231.3 kJ Include a negative sign, if appropriate. Do not include units in your response. Round your answer to two decimal places. Include a negative sign if appropriate. Provide your answer below: kJ


You'll get a detailed, step-by-step and expert verified solution.
 Work With Experts to Reach at Correct Answers
Work With Experts to Reach at Correct Answers