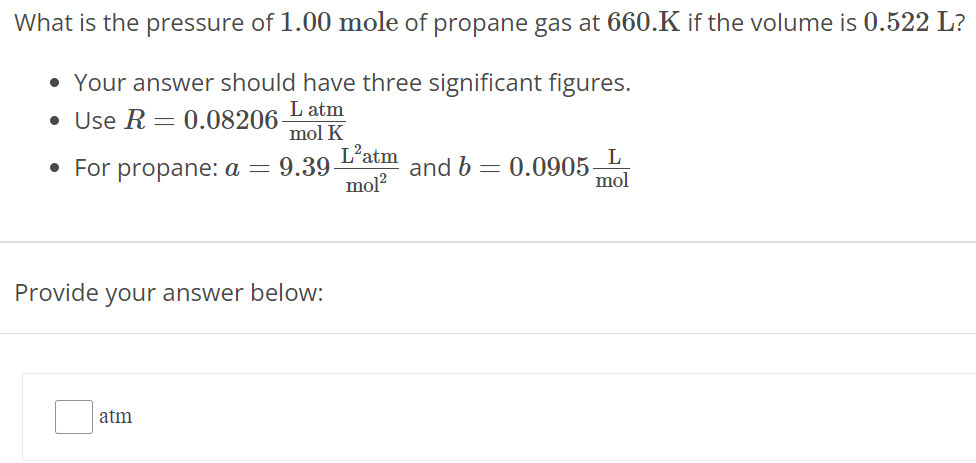
What is the pressure of 1.00 mole of propane gas at 660. K if the volume is 0.522 L ? Your answer should have three significant figures. Use R = 0.08206 L atm mol K For propane: a = 9.39 L2 atm mol2 and b = 0.0905 L mol Provide your answer below: atm