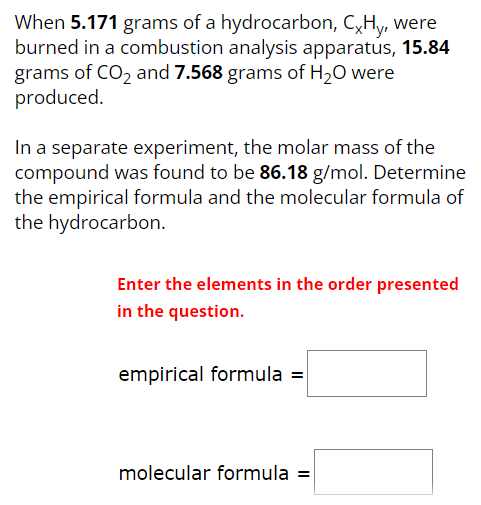
When 5.171 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 15.84 grams of CO2 and 7.568 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 86.18 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon. Enter the elements in the order presented in the question. empirical formula = molecular formula =