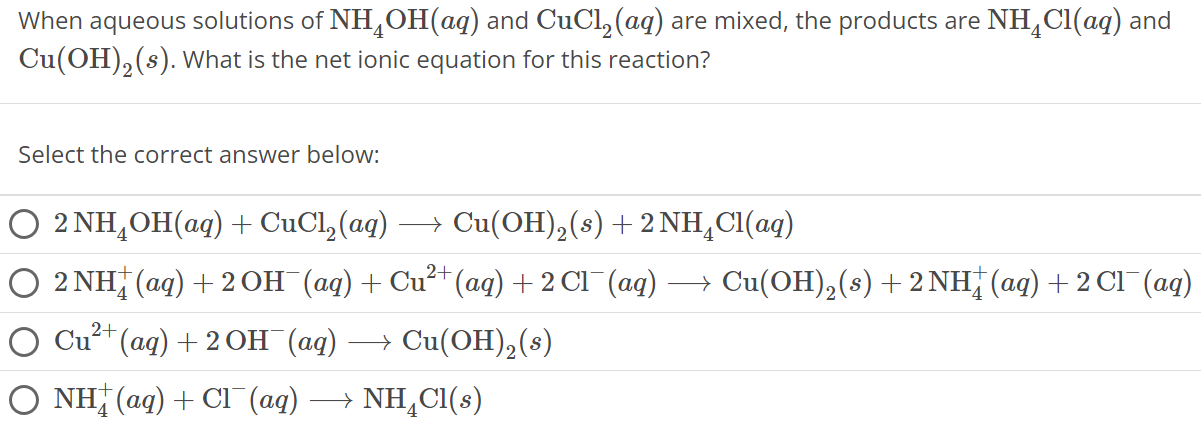When aqueous solutions of NH4 OH(aq) and CuCl2(aq) are mixed, the products are NH4 Cl(aq) and Cu(OH)2(s). What is the net ionic equation for this reaction? Select the correct answer below: 2NH4OH(aq) + CuCl2(aq) ⟶ Cu(OH)2(s) + 2NH4 Cl(aq) 2NH4+(aq) + 2OH−(aq) + Cu2+(aq) + 2Cl−(aq) ⟶ Cu(OH)2(s) + 2 NH4+(aq) + 2 Cl−(aq) Cu2+(aq) + 2OH−(aq) ⟶ Cu(OH)2(s) NH4+(aq) + Cl−(aq) ⟶ NH4 Cl(s)