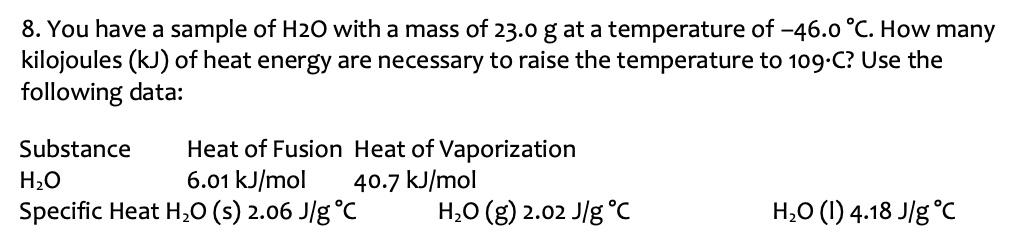You have a sample of H2O with a mass of 23.0 g at a temperature of −46.0∘C. How many kilojoules (kJ) of heat energy are necessary to raise the temperature to 109⋅C ? Use the following data: Substance Heat of Fusion Heat of Vaporization H2O 6.01 kJ/mol 40.7 kJ/mol Specific Heat H2O(s) 2.06 J/g∘ CH2O(g) 2.02 J/g∘ CH2O(I) 4.18 J/g∘C