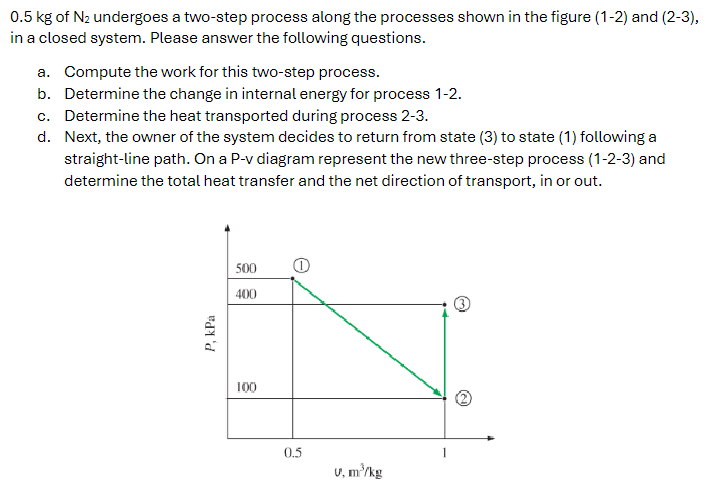
0.5 kg of N2 undergoes a two-step process along the processes shown in the figure (1-2) and (2-3), in a closed system. Please answer the following questions. a. Compute the work for this two-step process. b. Determine the change in internal energy for process 1-2. c. Determine the heat transported during process 2-3. d. Next, the owner of the system decides to return from state (3) to state (1) following a straight-line path. On a P-v diagram represent the new three-step process (1-2-3) and determine the total heat transfer and the net direction of transport, in or out.