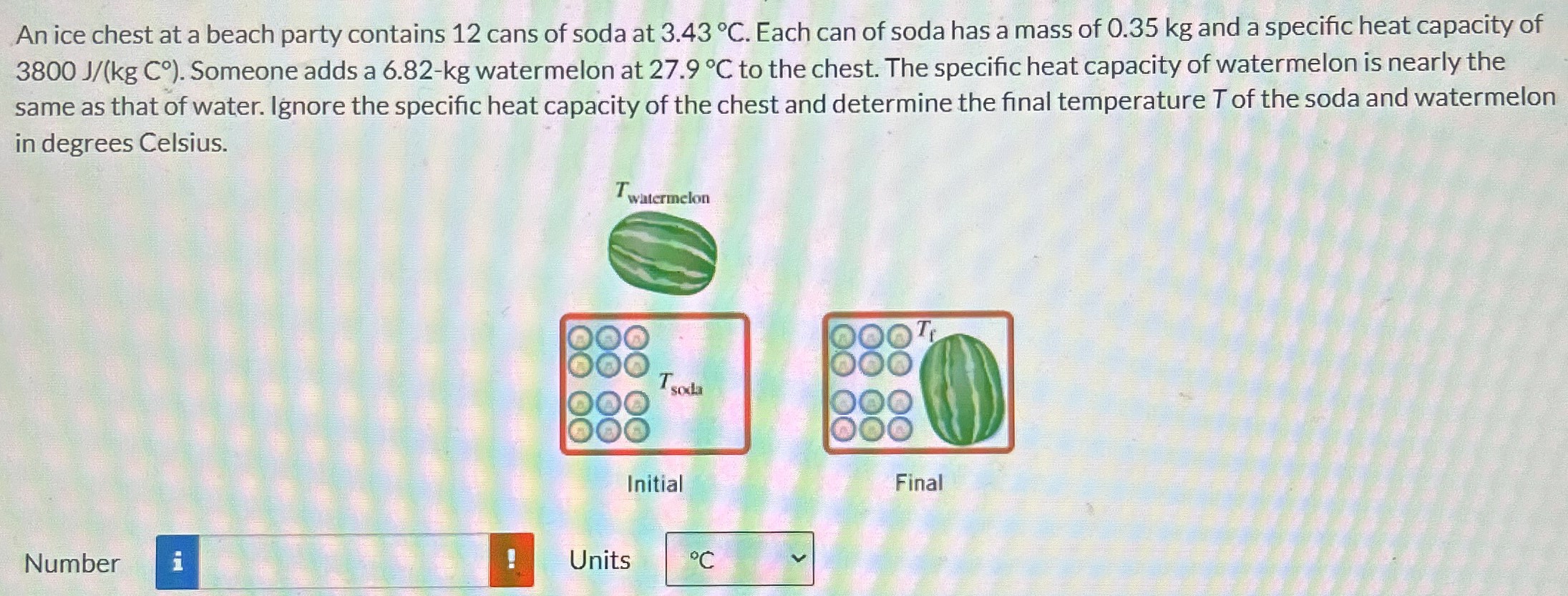
An ice chest at a beach party contains 12 cans of soda at 3.43∘C. Each can of soda has a mass of 0.35 kg and a specific heat capacity of 3800 J/(kgC∘). Someone adds a 6.82 - kg watermelon at 27.9∘C to the chest. The specific heat capacity of watermelon is nearly the same as that of water. Ignore the specific heat capacity of the chest and determine the final temperature T of the soda and watermelon in degrees Celsius. Initial Final Number Units