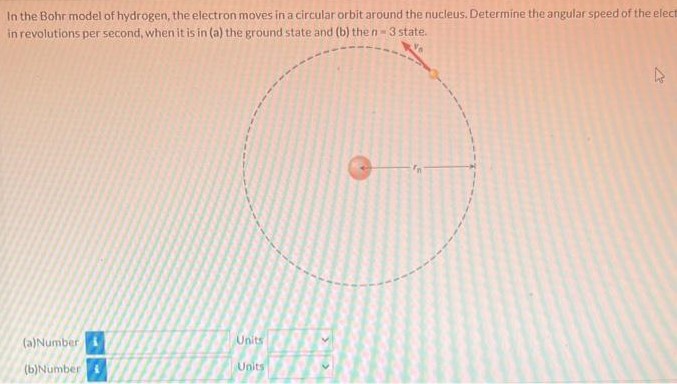
In the Bohr model of hydrogen, the electron moves in a circular orbit around the nucleus. Determine the angular speed of the electron In revolutions per second, when it is in (a) the ground state and (b) the n = 3 state. (a) Number Units (b) Number Units